
The US FDA New Drug Approvals in October 2024
Shots:
-
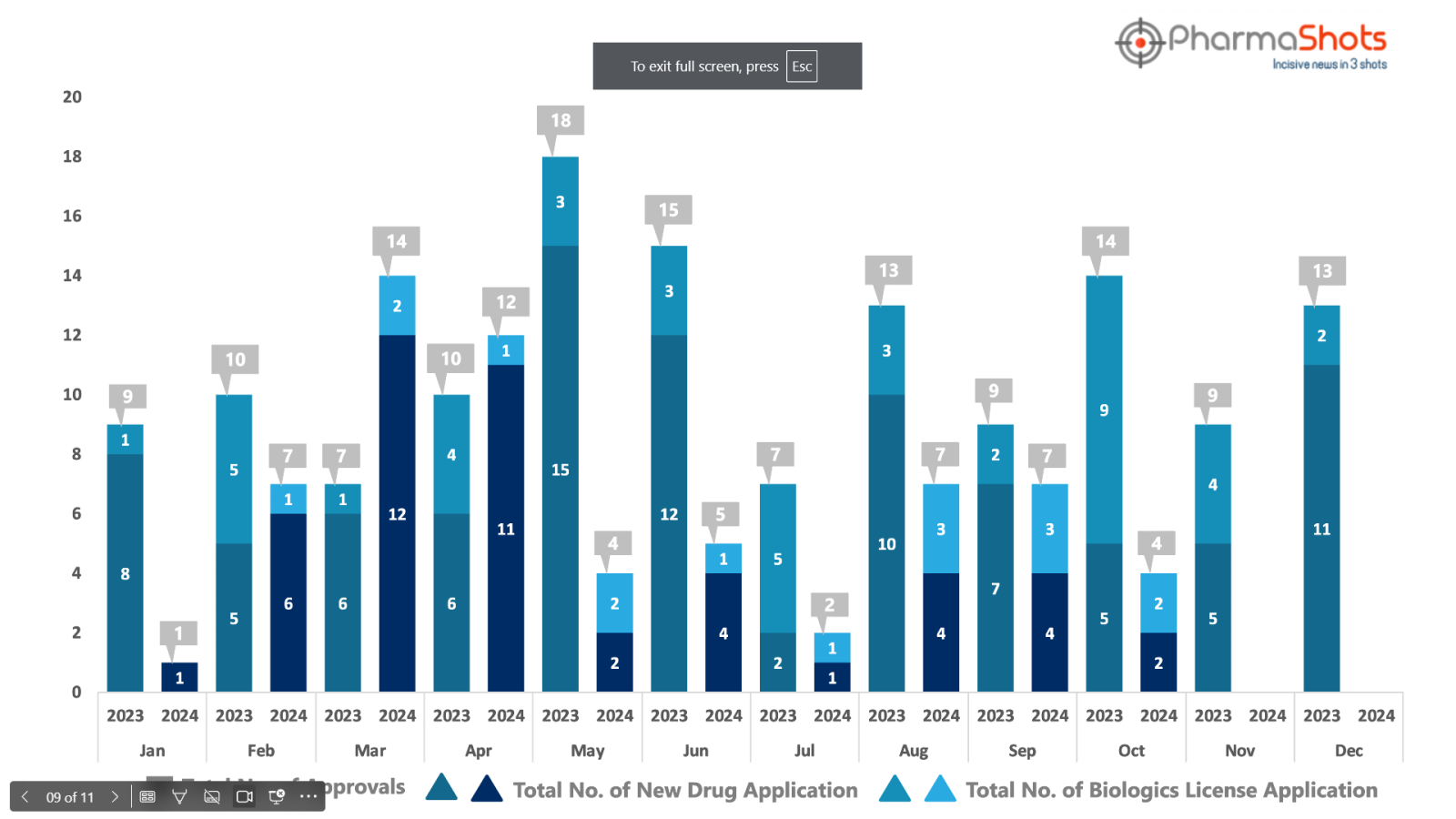
PharmaShots has compiled a list of US FDA-approved drugs in the month of October 2024
-
The US FDA has approved a total of 4 new drugs including 2 new molecular entities and 2 biologics leading to the treatment of patients and advances in the healthcare industry
-
The major highlighted drugs were Pfizer’s Hympavzi for the treatment of Hemophilia A & B
Product Name: Itovebi + Ibrance & fulvestrant
Active ingredient: Inavolisib
Company: Roche
Date: Oct 10, 2024
Disease: Breast Cancer
Shots:
-
The US FDA approved Itovebi + Ibrance & fulvestrant as a 1L treatment in adults with endocrine-resistant, PIK3CA-mutated, HR+ & HER2- locally advanced or metastatic breast cancer, based on the P-III (INAVO120) trial
-
P-III (INAVO120) trial assessed the safety and efficacy of Itovebi + Ibrance & fulvestrant vs PBO + Ibrance & fulvestrant (n= 325) and showed a 57% reduction in disease worsening or death vs palbociclib & fulvestrant alone as 1L treatment; the trial met its 1EP (PFS) & 2EPs (ORR & CBR) with OS follow-up continuing for the next analysis
-
Results from INAVO120 are also being used for submissions to other global health authorities. Additionally, the company is evaluating Itovebi in two more P-III trials (INAVO121 & INAVO122) for the same indication
Product Name: Hympavzi
Active ingredient: Marstacimab-hncq
Company: Pfizer
Date: Oct 11, 2024
Disease: Hemophilia A & B
Shots:
-
The US FDA has approved Hympavzi (QW, SC) as a prophylactic treatment to prevent bleeding episodes in patients (≥12yrs.) with hemophilia A & B without FVIII & FIX inhibitors, respectively. It has also received the CHMP’s positive opinion for the same
-
Results from pivotal P-III (BASIS) study, assessing Hympavzi in patients (12-75yrs.) with severe hemophilia A or mod. severe to severe hemophilia B with/without inhibitors, formed the basis of approval
-
Study depicted a reduction in the annualized bleeding rate (ABR) by 35% & 92% post 12mos. with Hympavzi vs routine prophylaxis & on-demand treatment. Safety was similar to the outcomes of P-I/II study, with most common AEs being injection site reactions, headache & pruritus
3. Astellas Reports the US FDA’s Approval of Vyloy (Zolbetuximab-clzb) to Treat Advanced G/GEJ Cancer
Product Name: Vyloy
Active ingredient: Zolbetuximab-clzb
Company: Astellas
Date: Oct 18, 2024
Disease: G/GEJ Cancer
Shots:
-
The US FDA has granted approval to Vyloy plus fluoropyrimidine & Pt-based CT as a 1L treatment of locally advanced unresectable or metastatic CLDN 18.2+, HER2-ve G/GEJ adenocarcinoma
-
Approval was based on the P-III (SPOTLIGHT & GLOW) studies assessing Vyloy + mFOLFOX6 and Vyloy + CAPOX, respectively, vs PBO as a 1L treatment of G/GEJ cancer. Astellas partnered with Roche to use Ventana CLDN18 (43-14A) RxDx Assay for identifying eligible patients
-
Results showed PFS of 24.9% (mPFS: 10.61mos. vs 8.67mos.) & OS of 25% (mOS: 18.23mos. vs 15.54mos.) in SPOTLIGHT as well as PFS of 31.3% (mPFS: 8.21mos. vs 6.8mos.) & OS of 22.9% (mOS: 14.39mos. vs 12.16mos.) in GLOW
4. Iterum Therapeutics’ Orlynvah (Oral Sulopenem) Receives the US FDA’s Approval to Treat uUTIs
Product Name: Orlynvah
Active ingredient: Sulopenem Etzadroxil & probenecid
Company: Iterum Therapeutics
Date: Oct 25, 2024
Disease: Uncomplicated Urinary Tract Infections (uUTIs)
Shots:
-
The US FDA has approved Orlynvah (sulopenem etzadroxil & probenecid) to treat uUTIs in adult women, specifically addressing infections caused by E.coli, Klebsiella pneumoniae, or Proteus mirabilis, particularly when there are few or no other oral therapy available
-
Approval was based on two pivotal P-III studies (SURE 1 & REASSURE) that assessed the safety & efficacy of Orlynvah vs ciprofloxacin (SURE 1) & Augmentin (REASSURE)
-
The SURE 1 trial found that Orlynvah was superior to ciprofloxacin for fluoroquinolone-resistant infections, while REASSURE demonstrated both non-inferiority & statistical superiority to Augmentin among patients susceptible to that antibiotic. Both trials indicated well-tolerability
Related Post: Insights+: The US FDA New Drug Approvals in September 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














